Introduction: Achievement of minimal residual disease negative (MRD-) status in multiple myeloma (MM) is associated with improved progression-free survival (PFS) and overall survival (OS). Isatuximab (Isa) is an approved anti-CD38 IgG kappa monoclonal antibody. We analyzed the depth of response including MRD-, long-term outcomes, and kinetics of tumor response in the IKEMA study. Measurement by mass spectrometry of serum M-protein was also performed to overcome the interference with Isa in standard immunofixation assay.
Methods: IKEMA was a randomized, open-label, multicenter Phase 3 study that investigated Isa plus carfilzomib and dexamethasone (Isa-Kd) vs Kd in patients (pts) with relapsed MM who received 1-3 lines of therapy (NCT03275285). The primary endpoint of PFS and secondary endpoints of overall response rate (ORR), very good partial response or better (≥VGPR) and complete response (CR) rate were determined by an Independent Response Committee based on central data for M-protein, central imaging review and local bone marrow for plasma cell infiltration according to IMWG criteria.MRD was assessed in bone marrow aspirates from pts who achieved ≥VGPR by next generation sequencing at 10-5 sensitivity level. Mass spectrometry analysis was performed to measure serum M-protein without Isa interference. Hazard ratios and corresponding confidences interval were estimated using Cox proportional hazards model. Secondary endpoints were compared between treatment arms using Cochran Mantel Haenszel test. Per ITT, all randomized pts not reaching MRD- or without MRD assessment were analyzed as MRD+.
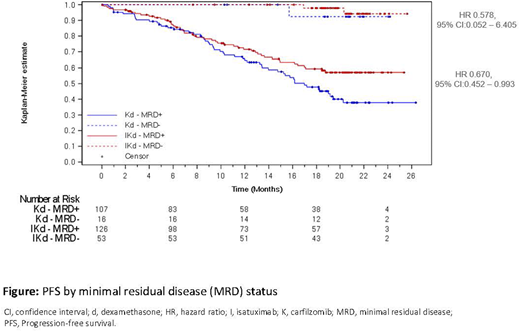
Results: 302 pts (179 Isa-Kd, 123 Kd) were randomized. At a median follow-up of 20.7 months deeper responses were observed in Isa-Kd vs Kd with ≥VGPR 72.6% vs 56.1% (nominal p=0.011) and ≥CR 39.7% vs 27.6%, respectively. MRD- occurred in 53/179 (30%) pts in the Isa-Kd arm vs 16/123 (13%) in the Kd arm (nominal p=0.0004) with 20.1% (36/179 pts Isa-Kd) vs 10.6% (13/123 pts Kd) reaching CR and MRD-. PFS by MRD status is shown in the Figure, HR favors Isa-Kd vs Kd in both MRD- pts (HR 0.578, 95%CI: 0.052-6.405) and MRD+ pts (HR 0.670, 95% CI: 0.452-0.993). MRD- pts had a longer PFS than MRD+ pts. Within Isa-Kd, MRD-negative status could be obtained in pts with renal impairment (26.5% MRD- vs 25.9% MRD+ with eGFR <60mL/min/1.73 m2); with ISS stage III at diagnosis (32.1% MRD- vs 27.8% MRD+); with t(4;14) [13.2% MRD- vs 11.9% MRD+], with gain(1q21) [45.3% MRD- vs 40.5% MRD+]; in heavily pretreated ≥3 prior lines (22.6% MRD- vs 19.0% MRD+) or refractory to lenalidomide in last regimen (18.9% MRD- vs 20.6% MRD+). Within Isa-Kd, MRD-negative status was reached less frequently in pts refractory to a proteasome inhibitor (PI) [18.9% MRD-vs 36.5% MRD+) or with del(17p), [3.8% MRD- vs 12.7% MRD+].
Interference of Isa with M protein was explored: samples from 27 pts with near-CR (only serum immunofixation (IF) positive IgG kappa) or potential CR (serum remaining M-protein ≤ 0.5 g/dL with IF positive IgG kappa) in the Isa-Kd arm were tested by mass spectrometry. Among them, 11 near CR or potential CR pts had documented <5% plasma cells in bone marrow and were mass spectrometry negative (residual myeloma M-protein level below LOQ of central lab immunofixation). In addition, 7/11 were also MRD-. These results support that both current CR rate and MRD- CR rate are underestimated (potential adjusted CR rate of 45.8%; potential adjusted MRD- CR rate 24%). Responses occurred quickly in both arms. The median time (Isa-Kd vs Kd) in responders to: first response was 32.0 (28-259) days vs 33.0 (27-251) days; best response 120.0 (29-568) days vs 104.5 (29-507) days; first CR 184.0 (30-568) days vs 229.5 (58-507) days; first ≥VGPR 88.0 (28-432) vs 90.0 (29-491) days, respectively. In addition to increased depth of response, quality of life as measured by QLQ-C30 Global Health Status scores was maintained with Isa-Kd per descriptive analyses.
Conclusions: There was a clinically meaningful improvement in depth of response in Isa-Kd vs Kd. CR rate in Isa-Kd of 39.7% was underestimated due to interference. Mass spectrometry results suggest that the potential adjusted CR rate could be reached for 45.8% of pts with 1 to 3 prior lines treated in Isa-Kd. More pts in Isa-Kd vs Kd reached MRD negativity (30% vs 13%) and at least twice as many reached CR MRD- (20.1% vs 10.6%; adjusted 24% vs 10.6%). Reaching MRD negativity was associated with longer PFS in both arms.
Martin:Legend Biotech: Consultancy; Sanofi, Amgen, Seattle Genetics, JNJ - Janssen: Research Funding. Mikhael:Amgen, Celgene, GSK, Janssen, Karyopharm, Sanofi, Takeda: Honoraria. Hajek:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharma MAR: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Oncopeptides: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding. Kim:Amgen, BMS, Janssen, Sanofi, Takeda: Consultancy, Honoraria, Research Funding. Suzuki:Takeda, Celgene, ONO, Amgen, Novartis, Sanofi, Bristol-Myers Squibb, AbbVie and Janssen: Honoraria; Bristol-Myers Squibb, Celgene and Amgen: Research Funding; Takeda, Amgen, Janssen and Celgene: Consultancy. Hulin:Celgene/Bristol-Myers Squibb, Janssen, GlaxoSmithKline, and Takeda: Honoraria. Garg:Janssen, Takeda, Celgene, Novartis, Sanofi: Honoraria. Quach:GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Consultancy; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Honoraria; Amgen, Celgene, karyopharm, GSK, Janssen Cilag, Sanofi.: Membership on an entity's Board of Directors or advisory committees; Amgen, sanofi, celgene, Karyopharm, GSK: Research Funding. Risse:Sanofi: Current Employment. Asset:Sanofi: Current Employment. Macé:Sanofi: Current Employment. van de Velde:Sanofi: Current Employment, Current equity holder in publicly-traded company. Moreau:Janssen: Consultancy, Honoraria; Novartis: Honoraria; Takeda: Honoraria; Abbvie: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria.
Isatuximab, a monoclonal CD38 antibody, is approved in combination with pomalidomide and dexamethasone in the United States, the European Union, Canada, Australia, Switzerland, and Japan for the treatment of adult patients with relapsed/refractory multiple myeloma who have received at least two prior therapies, including lenalidomide and a proteasome inhibitor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal